DEAS has been producing medical devices for respiratory care since 1989 under the DEAFLUX® brand name in its factory in Via dell’Industria in Castel Bolognese.
All core activities – such as designing, moulds manufacture, extrusion, injection and blow moulding – are carried out in our productive departments with strict quality controls, to guarantee high reliability and service standards.
The competency acquired in forming thermoplastics, associated with an active research work and spirit of innovation, allow us to provide every breathing solution on an OEM basis as well, in compliance with EU Regulation No. 2017/745 (MDR).
At its plant in Via Lughese in Castel Bolognese, DEAS produces medical recording papers under the EasyTrace® brand. Since 1980 the EasyTrace® brand has identified this product line worldwide.
It includes every model of medical recording paper for Electrocardiograms, Foetal monitoring, Neurology, Gynaecology, Pneumology, Intensive Care Units, Resuscitation, Ultrasound scans, and Laboratory analysis.


DEAS è fondata da Domenico Scardovi, Davide Scardovi e Augusto Dall’Arno.

DEAS crea il marchio DEAFLUX ed inizia a sviluppare e a produrre dispositivi per terapia respiratoria.

DEAS apre l’officina per la produzione di stampi ad iniezione.

DEAS trasferisce gli impianti in un nuovo stabilimento per aumentare la sua capacità produttiva e di stoccaggio.

DEAS ottiene la certificazione del proprio sistema di qualità secondo la
EN ISO 13485.

DEAS ottiene la certificazione di processo secondo la EN ISO 14001 sul controllo degli impatti ambientali delle proprie attività.

DEAS apre il reparto per lo sviluppo e la produzione di apparecchi elettromedicali.

L’umidificatore respiratorio Hydraltis viene lanciato sul mercato.
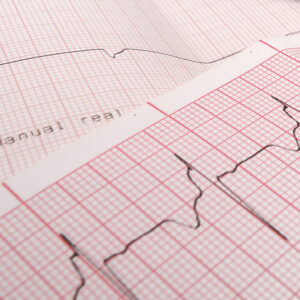
DEAS incorpora Modul Diagram e acquisisce il marchio EasyTrace.

DEAS adotta un codice etico.